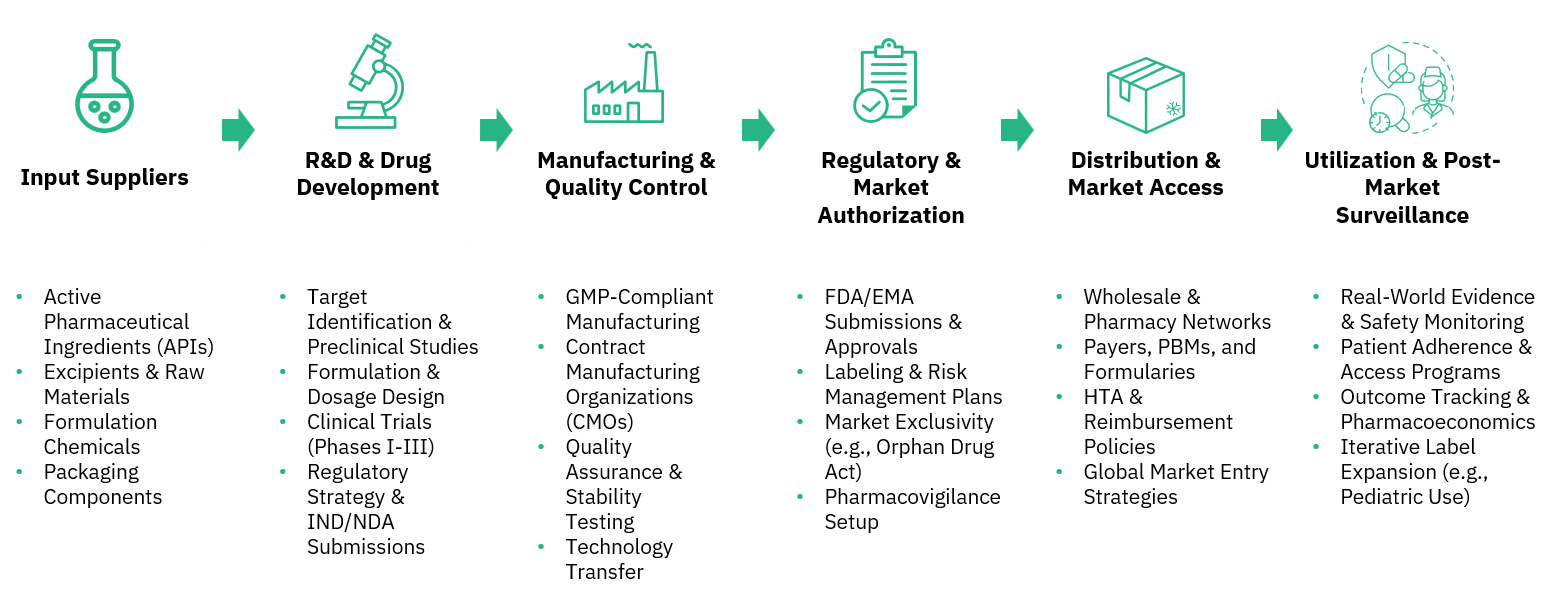
Category Drug Market Intelligence
The pharmaceutical drugs are evolving rapidly in response to shifting global disease burdens, patient preferences, and payer priorities. From precision driven orphan drug breakthroughs to consumer driven OTC choices, this cluster captures diverse drug classes redefining how care is delivered, accessed, and personalized.
Backed by clinical relevance and regulatory support, these drugs are central to public health, chronic disease management, pediatric care, and self medication. The rise of digital pharmacies, AI guided repurposing, and decentralized clinical research is pushing this market into its next evolution.

What’s Driving Momentum?
Consumer empowerment in self care and OTC drug usage post COVID
Surging orphan drug approvals under expedited regulatory pathways (e.g., US FDA, EMA PRIME)
Personalized medicine boom aligned with pharmacogenomics and digital biomarkers
Pediatric reform incentives pushing R&D in child specific formulations (NIH, FDA Safety & Innovation Act)
Generic drug penetration into biosimilars and complex generics with cost effective access strategies
Nutraceutical pharma convergence redefining preventive care boundaries
At the same time, developers are grappling with scale up inefficiencies, price sensitivity, and growing complex regulatory pathways. That’s where we come in!
How we help:
From fast evolving OTC trends to the complexities of orphan drug access and generic pricing pressures, the category drugs division demands more than basic data. We deliver strategic insights that help clients identify high growth segments, decode regulatory shifts, and stay ahead of consumer and payer behavior. Whether you're planning a product launch or reshaping your market approach, we help turn insights into action across every therapeutic niche.